sodium bicarbonate hazards
Sodium bicarbonate IUPAC name. The latest Lifestyle Daily Life news tips opinion and advice from The Sydney Morning Herald covering life and relationships beauty fashion health wellbeing.
 |
| Pdf Sodium Bicarbonate Containing Mouthwash For Preventing Radiotherapy Induced Oral Mucositis In Patients With Locally Advanced Head And Neck Cancer |
Hydrogen ion H known as a BrønstedLowry acid or forming a covalent bond with an electron pair known as a Lewis acid.

. Potassium hydroxide is also a precursor to other potassium. Few Uses of Sodium Carbonate are Listed Below. ACEP Member Login. Soln of sodium hypochlorite containing 045-050 g of the salt in 100 ml.
May be prepared also from 154 g chlorinated lime 30 available chlorine 7. AI May Come to the Rescue of Future Firefighters. This reduced level of sodium bicarbonate NaHCO 3 15 gL is intended for use in a 5 CO 2 in air. Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions.
Sds_71111 Sodium Bisulfite Starch Solution for AP6188. NIST Study Finds Wildfire Hazards in Residential Fences and Mulch Beds. It is not intended for use on an out-of-control fire such as one which has reached the ceiling endangers the user ie no escape route smoke explosion hazard etc or otherwise requires the equipment personnel. With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl.
Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. Breathing ammonium bicarbonate can irritate the nose throat and lungs causing coughing wheezing andor shortness of breath. Short-term health effects may occur immediately or shortly after exposure to ammonium bicarbonate. This website provides easy access to all the pesticide-related information that is contained in various pesticide topical sites.
Ammonium bicarbonate is an irritant to the skin eyes and respiratory system. 58 Monday March 26 2012 Rules and Regulations 09132019 EN English US 56 Sodium Bicarbonate 144-55-8 LC50 fish 1 8250 - 9000 mgl EC50 Daphnia 1 2350 mgl. 2NaHCO 3 Na 2 CO 3 CO 2 H 2 O. Sodium ascorbate normally provides 131 mg of sodium per 1000 mg of ascorbic acid 1000 mg of sodium ascorbate contains 889 mg of ascorbic acid and 111 mg of sodium.
It is a white water-soluble solid that serves as a buffering and chelating agent with many applications in the food industryWhen crystallized from water it forms a hexahydrate but it dehydrates above room temperature. Prepd by diluting with distilled water a soln of sodium hypochlorite adding a 5 soln of sodium bicarbonate nad adjusting to proper strength and concn according to procedure described in NF. Sodium nitrate is a white deliquescent solid very soluble in. When building fences and landscaping their properties homeowners should keep fire safety on the top of their minds.
Disodium pyrophosphate or sodium acid pyrophosphate SAPP is an inorganic compound consisting of sodium cations and pyrophosphate anion. Comments This reduced level of sodium bicarbonate NaHCO3 15 gL is intended for use in 5 CO 2 in air. The first category of acids are the proton donors or BrønstedLowry acidsIn the special case of aqueous solutions proton donors form the hydronium ion H 3 O and are known as Arrhenius. 201-796-7100 For CHEMTREC assistance call.
A fire extinguisher is a handheld active fire protection device usually filled with a dry or wet chemical used to extinguish or control small fires often in emergencies. Sodium carbonate Na 2 C O 3 also known as washing soda soda ash and soda crystals is the inorganic compound with the formula Na 2 CO 3 and its various hydrates. Sodium nitrate is the chemical compound with the formula Na N O 3This alkali metal nitrate salt is also known as Chile saltpeter large deposits of which were historically mined in Chile to distinguish it from ordinary saltpeter potassium nitrateThe mineral form is also known as nitratine nitratite or soda niter. Sodium chloride is the salt most.
In order to buy non-prescription medicines you must be a registered user of our site as we are obliged to record your transaction history. Fisher Scientific 1 Reagent Lane Fair Lawn NJ 07410 For information call. Sodium bicarbonate is used in the treatment of metabolic acidosis associated with many conditions including severe renal disease eg renal tubular acidosis uncontrolled diabetes ketoacidosis extracorporeal circulation of the blood cardiac arrest circulatory insufficiency caused by shock or severe dehydration ureterosigmoidostomy lactic acidosis alcoholic. Sodium hydrogencarbonate commonly known as baking soda or bicarbonate of soda is a chemical compound with the formula NaHCO 3It is a salt composed of a sodium cation Na and a bicarbonate anion HCO 3 Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powderIt has a slightly salty.
Historically it was extracted from the ashes of plants growing in sodium-rich soils. Use your society credentials to access all journal content and features. Flinn Safety Data Sheets SDS contain valuable information about potential chemical hazards chemical composition proper storage and handling recommended disposal and personal protection information. The Sodium biCarbonate is then heated to make Na2CO3.
It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. ACEP Members full access to the journal is a member benefit. It also includes news and meeting information an A-Z index and more. We also ask that you complete our questionnaire so our pharmacy team can check that this product is suitable for you to buy.
Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly. Sodium hypochlorite commonly known in a dilute solution as bleach is an inorganic chemical compound with the formula NaOCl or NaClO comprising a sodium cation Na and a hypochlorite anion OCl or ClO It may also be viewed as the sodium salt of hypochlorous acidThe anhydrous compound is unstable and may decompose explosively. When anhydrous Sodium Carbonate is dissolved in water it recrystallizes to create washing Soda crystals that contain 10 molecules of water. All forms are white odourless water-soluble salts that yield moderately alkaline solutions in water.
Sodium ascorbate is produced by dissolving ascorbic acid in water and adding an equivalent amount of sodium bicarbonate in water. Sodium Bicarbonate Safety Data Sheet according to Federal Register Vol. Hazards NFPA 704 fire diamond 0. An acid is a molecule or ion capable of either donating a proton ie.
Additional sodium bicarbonate may be required for use in incubators containing higher percentages of CO 2. It is possible to recycle the CO2 gas produced. Potassium hydroxide also known as lye is an inorganic compound with the chemical formula KOHAlso commonly referred to as caustic potash it is a potent base that is marketed in several forms including pellets flakes and powdersIt is used in various chemical industrial and manufacturing applications. Sodium acetate is also used in heating pads hand warmers and hot iceSodium acetate trihydrate crystals melt at 58584 C 13641371 F dissolving in their water of crystallizationWhen they are heated past the melting point and subsequently allowed to cool the aqueous solution becomes supersaturatedThis solution is capable of cooling to room.
 |
| Safety With Sodium Bicarbonate Velocityehs |
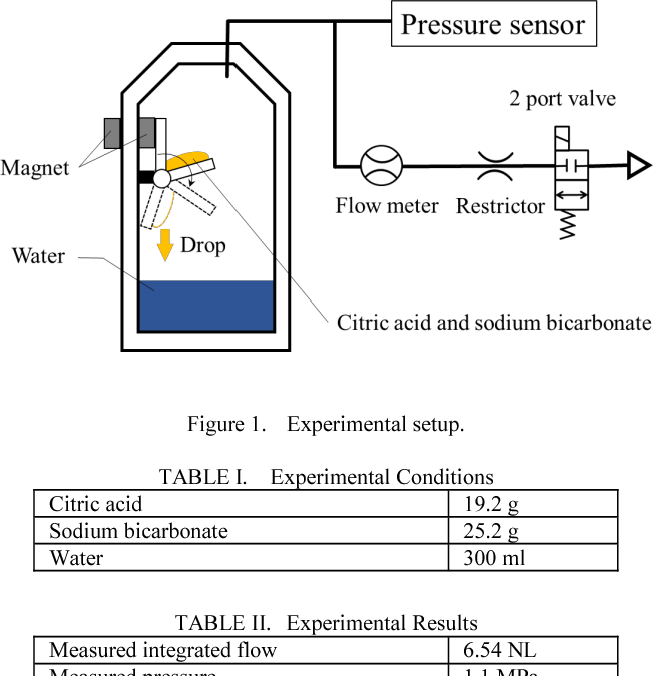 |
| A Pneumatic Power Source Using A Sodium Bicarbonate And Citric Acid Reaction With Pressure Booster For Use In Mobile Devices Semantic Scholar |
 |
| Sodium Bicarbonate Wikipedia |
 |
| Msds Sodium Bicarbonate Australian Mud Santos |
 |
| Sodium Bicarbonate Production From Soda Ash Ppt Download |
Posting Komentar untuk "sodium bicarbonate hazards"